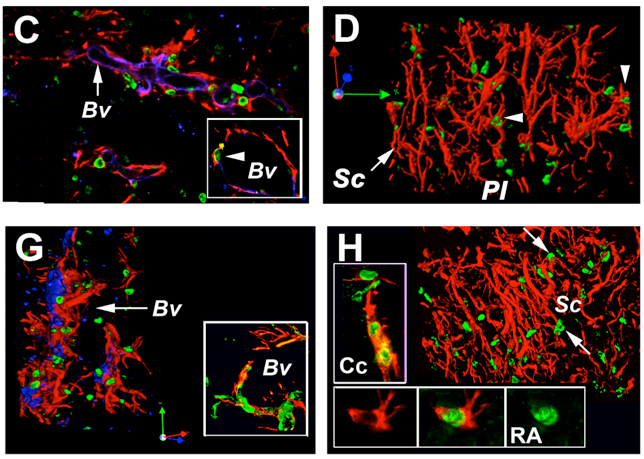
We work mainly with floating sections but occasionally also with paraffin sections. After stroke neuroepithelial cells contributing to the glial (D, H) scar emanate from desintegrated the blood vessel walls; Green: BrdU nuclei; Violett: Laminin (Immunostaining using floating sections)
Global measurement of proteome turnover by isotopic labeling of mice
Proper proteome dynamics is critical to normal development and aging. In order to avoid aging, the cell structure within tissues must be maintained by a continuous synthesis and degradation of worn-out molecules process, ie a continuous turnover process. Efficient macromolecular turnover is integral to the normal function and survival of a biological system. Although there are large variations in the rates of degradation of individual proteins, it is generally observed that overall protein turnover slows down during aging. The physiological consequences of decreased protein turnover include the accumulation of altered and abnormal proteins, an altered pattern of posttranslational modifications due to increased dwell time and a disruption of the organisation of the cytoskeleton and extracellular matrix.
Currently we investigate the age-associated changes in brain, liver, heart and kidney protein turnover in mice fed 15N-labeled blue-green algae (Spirulina platensis). Our cooperating partner, Dr. Ghaemmaghami of the Rochester University, USA, has recently succeeded in isotopically labeling mice using a diet supplemented with 15N-enriched, blue-green algae (Spirulina platensis). 15N-labeled salts are used as the sole nitrogen source for the Spirulina cultures, resulting in >99% isotopic enrichment. The labeled algae is used as the sole protein source in the diet of mice. Using this approach, the degree of labeling over time can be used to measure protein turnover rates. The measured rates of turnover spanned four orders of magnitude, from 0.002 d-1 to 10 d-1. The distribution of the turnover rates in the brain is skewed toward long-lived proteins whereas the distributions of the blood and liver are skewed towards relatively faster turnover rates. The median turnover rate for the brain was 0.075 d-1 compared to 0.23 and 0.20 d-1 for the blood and liver. Thus, the average lifetime of proteins in the brain, liver and blood are 9.0, 3.0 and 3.5 days respectively. In a number of examples, evidence for the regulation of turnover at the level of protein complex, organelle and tissues was provided (Price et al., 2010).
References
Price JC, Guan S, Burlingame A, Prusiner SB, Ghaemmaghami S (2010). Analysis of proteome dynamics in the mouse brain. Proc Natl Acad Sci U S A.107: 14508-13.